The RGC Effect: A Decade in the Making
Reflecting on the first 10 years of the Regeneron Genetics Center® (RGC), with impact far beyond what we could have imagined
February 5, 2024Revolutionizing drug discovery
Successful new medicines are more the exception than the rule: Over 90% of new therapies that enter clinical trials fail, costing billions of dollars in the process.1,2 The awesome power of human genetics to rewrite the “rules” of drug discovery centers on genes that are known to cause or protect from disease, potentially becoming the difference between an unsuccessful drug candidate and a life-changing therapy.
Prior to the inception of Regeneron Genetics Center® (RGC), Regeneron had only experienced the power of harnessing the human genome in a handful of programs. Discovery efforts at the time were greatly limited by the ability to conduct sequencing and analysis at the scale needed for broad advancements. In 2013, we set out on the ambitious initiative to sequence 100,000 people, with the goal of identifying genetic targets associated with disease. We hoped to find maybe a few genetic targets that could, if we were lucky, lead us to one new medicine.
Looking back, our progress and success have far exceeded our initial goals and dreams.
Our work has led to the discovery of over 50 new protective variants across disease types including obesity, chronic obstructive pulmonary disease (COPD), cancer and blinding eye diseases.3-6 Along with our colleagues in Regeneron’s research and preclinical development team, we have advanced around 30 new drug targets into our development pipeline, with several programs now in the clinic.
RGC’s efforts and expertise have also extended beyond genetic drug targets. We have found new genetic associations with disease risk and progression,7-9 and we support wider clinical development in identifying new drug indications4 and aiding in trial design,10 bolstering new drug development and disease treatment every step of the way.
Our productivity and success in new genetic target discovery has led Regeneron to investments in genetic medicine platforms both internally and through partnerships, moving us beyond the realm of antibodies in applying genetic discoveries to therapeutic development.
But we haven’t stopped there.
We are well on our way to creating the largest and most comprehensive genomic database in the world, with more than 2.3 million people sequenced to date and a goal of reaching 5–10 million in the near future. As part of this endeavor, we recognize the critical role of genomic diversity: We have sequenced over 500,000 people of non-European ancestries, with a commitment to reaching millions of people of diverse ancestries and environments.
Keys to success
Our initial ideas and assumptions, while risky, were simple and evidence based. We were searching for genes with a large impact on human disease, so we knew we needed to focus on massive-scale sample sizes and rare gene variants. Accordingly, we prioritized exome sequencing and imputed genomes as the best approach to efficiently and economically achieve the largest sample sizes possible.
Our values have also been instrumental in our success. Innovation and collaboration have been a priority from the beginning. We have invested heavily in the best people and the most cutting-edge technologies to ensure that we have the necessary in-house expertise. We have also fostered and invested in collaborations with over 130 organizations, giving us the ability to tap into volunteer participants around the world.
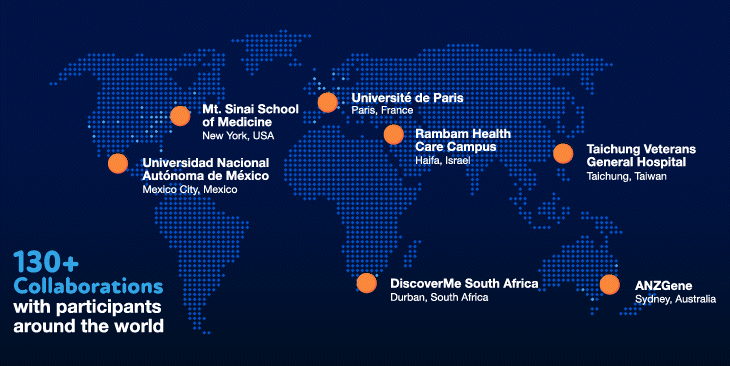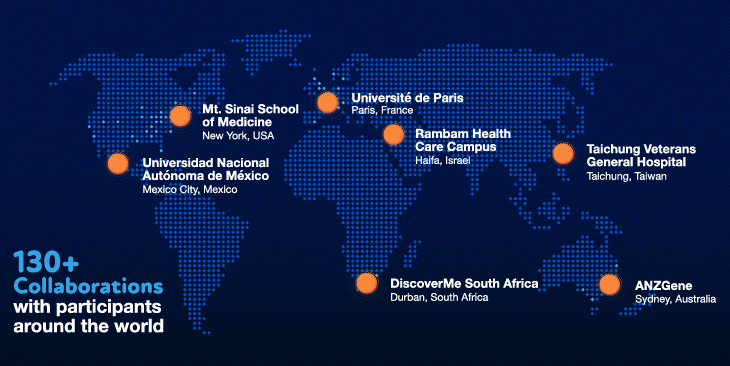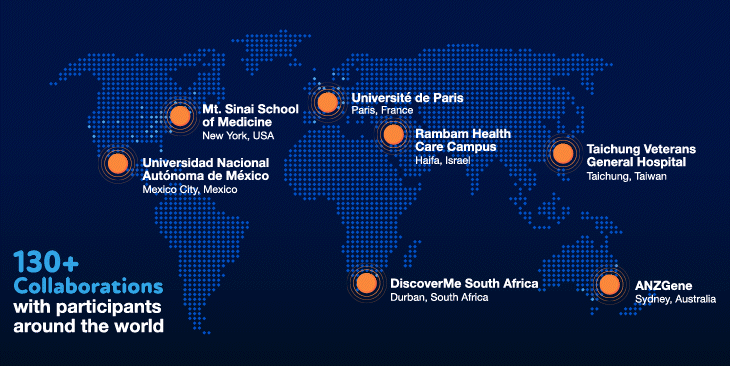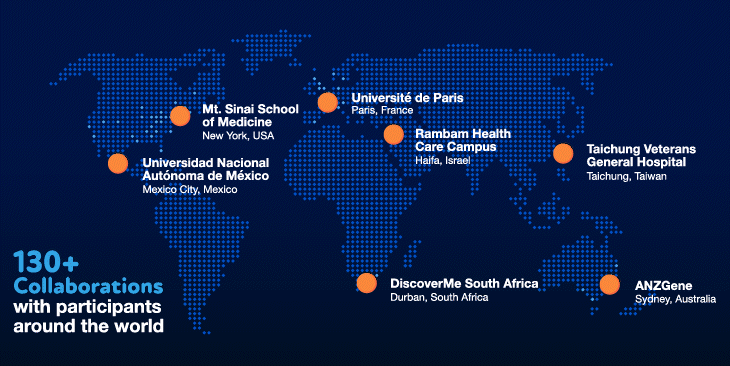
We have certainly hit our speedbumps on the road to scientific innovation. As with many initiatives at Regeneron, however, failures and bottlenecks were embraced and learned from, and were the substrate for new ideas and inventions that allowed us to become world leaders and experts in human genetics. Shared values of teamwork, perseverance and humility — and the willingness to make special efforts to go above and beyond for a greater collective purpose — have shaped our approach and served us well in our journey.
In some ways, the perspectives shaping our future are still the same simple, scientifically grounded ideas we formulated over 10 years ago regarding the power of human genetics in understanding health and disease to fuel drug discovery and development. Although we have been able to study dozens of diseases at a level that is exciting and productive, we still need to amass more data with even larger samples sizes to study hundreds of other diseases.
We believe that understanding human genetics is the first step to unlocking the predictive power and treatment potential within human biology, and we are committed to furthering this mission. If anything, our work thus far has shown us that the power of the human genome is far greater than we ever imagined. And this is just the beginning.
- Sun D, Gao W, Hu H, Zhou S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm Sin B. 2022;12(7):3049-3062.
- Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018 [published correction appears in JAMA. 2022 Sep 20;328(11):1110] [published correction appears in JAMA. 2022 Sep 20;328(11):1111]. JAMA. 2020;323(9):844-853.
- Akbari P, Gilani A, Sosina O, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373(6550):eabf8683.
- Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med. 2021;9(11):1288-1298.
- Kessler MD, Damask A, O'Keeffe S, et al. Common and rare variant associations with clonal haematopoiesis phenotypes. [published correction appears in Nature. 2023 Mar;615(7950):E3]. Nature. 2022;612(7939):301-309.
- Praveen K, Patel GC, Gurski L, et al. ANGPTL7, a therapeutic target for increased intraocular pressure and glaucoma. Commun Biol. 2022;5(1):1051.
- Damask A, Steg PG, Schwartz GG, et al. Patients with high genome-wide polygenic risk scores for coronary artery disease may receive greater clinical benefit from alirocumab treatment in the ODYSSEY OUTCOMES Trial. Circulation. 2020;141(8):624-636.
- Horowitz JE, Kosmicki JA, Damask A, et al. Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nat Genet. 2022;54(4):382-392.
- Kember RL, Merikangas AK, Verma SS, et al. Polygenic risk of psychiatric disorders exhibits cross-trait associations in electronic health record data from European ancestry individuals. Biol Psychiatry. 2021;89(3):236-245.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107.

